UV Smart D60

D60

See how D60 works in hospital
CE Certificate
The D60 received CE certification in April 2022 (MDR Class IIa).
FDA
The D60 is registered at the FDA as a class II medical device.
Standards
Our product complies with NEN-EN 60601-1 (Medical electrical equipment – General requirements for basic safety and essential performance – Collateral standard: Electromagnetic disturbances – Requirements and tests). This certificate indicates that the D60 complies with the general safety requirements for medical electrical equipment.
The EN 14885 standard is used as a reference for the D60 (Chemical disinfectants and antiseptics – Application of European Standards for chemical disinfectants and antiseptics). This certification means that the equipment achieves the same level of disinfection as wipes and liquids (as there is currently no guideline for UV-C).
UV Smart Technologies B.V. has held the NEN-EN-ISO 13485 certificate since 2020. This certification is the international standard for manufacturers, suppliers and distributors of medical devices to demonstrate that they meet the legal requirements for medical devices.
Patent protection
Impelux™ is a patented technology that uses UV-C for guaranteed disinfection without damaging equipment and with validated and continuous effectiveness.
Technical specifications
Channel-less endoscopes and TEE-probes
Dimensions
Width: 861 mm
Depth: 473 mm
Height: 2033 mm
Power cable
Length: 2 m
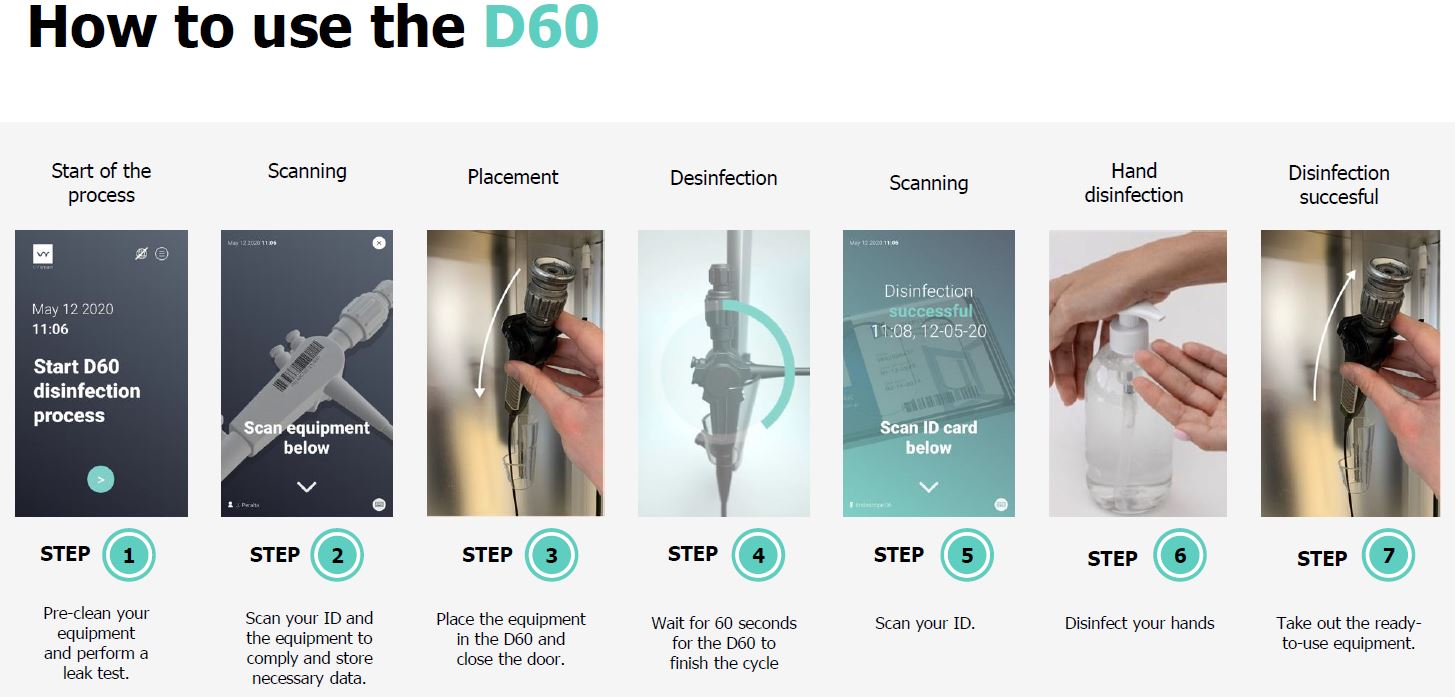
Disinfection cycle
60 seconds
Effective against
Bacteria, spores, yeast, mold, mycobacteria and viruses
Compatibility
Compatibility tests were performed on several products from Pentax, Olympus, Xion and Karl Storz with the D60 to verify the impact of UV-C light on endoscope materials. The conclusion is very positive and the D60 has therefore received multiple compatibility approvals.
Clinical evidence
The disinfection efficiency of the UV Smart D60 has been validated and proven by extensive research conducted in medical centers such as: Spaarne Gasthuis (Netherlands), Marburg University (Germany) and UZ Gent (Belgium). Studies have also been conducted in clinical laboratories (and institutions), including Streeklab Haarlem (Netherlands), and Eurofins (Italy).
Eurofins and Streekklab Haarlem*
Eurofins and the Streekklab concluded that D60 meets the European standard for cleaning and disinfection of medical devices. Conclusion: D60 meets the NEN-EN-14885:2018 standards for the full spectrum of microorganisms.
Spaarne Gasthuis*
Spaarne Gasthuis compared the D60 with standard disinfection washers. The conclusion: the first part of the laboratory study showed that the D60 meets the reduction requirements for S. aureus, C. albicans, S. pneumoniae, B.subtilis and E.coli. The second part of this study found that the D60, on average, showed a visible improvement in terms of disinfection compared to the current method: the disinfection washer.
Marburg University*
Following favorable results of disinfection with rigid endoscopes using Impelux™ technology, Marburg University investigated the effectiveness of disinfection of flexible endoscopes without a working channel with the D60. The study concluded that the D60 efficiently reduces bacterial contamination of flexible endoscopes.
UZ Gent*
UZ Gent investigated the effectiveness of the disinfection of TEE probes in the D60. The conclusion: A clear reduction of the number of CFU is noticeable after disinfection with the D60. The handle contains noticeably fewer germs after disinfection with the D60 compared to the Soluscope.
*Research is available on request

